Exploring Molecular Shapes
Exploring Molecular Shapes
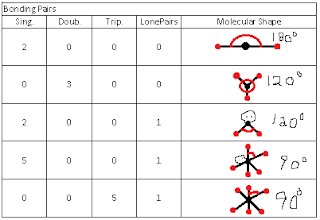
The question we were trying to answer was "How does the number of things around a central atom affect the shape of a molecule?" We care about the shape of the molecule because it helps determine the properties of the molecule. During our investigation we played with a online simulator that allowed us to put different combinations of bonds and lone electron pairs around a central atom. We made a table of the data we obtained, containing the types of bonds they had and the molecular structure of each one.
Our claim was, as more bonds are added it changes the shape and degree of the molecule.
Evidence:
Reasoning: VSEPR is a model that any given pair of valence shell strives to get as far away as possible from all the other electron pairs in the shell. Bonded atoms and unbonded electron pairs will affect the shape the same way because they will have the same amount of things around the molecule based on the molecular shape.

Comments
Post a Comment