Exploring Isotopes & Average Atomic Mass
Exploring Isotopes & Average Atomic Mass
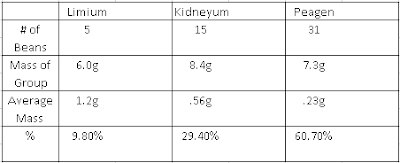
The question we were trying to answer was "What is the atomic mass of beanium". During our investigation we separated the three different types of beanium, counted them, and then took the mass of each type in groups. We weighed it this way because each bean is not same, but we want them to be. We got the mass of each group and divided it by the number of beans to get the mass of an individual bean.
Our claim was that the atomic mass of beanium is .42g.
Evidence:
Our reasoning is we took the average mass of bean and multiplied it by the abundance(percent) and added each one than divided by 100. for this we got the atomic mass of beanium as .42g. We took the average mass of the beans because we want them to weigh the same. We took the percent because there isn't the same amount of beans per group.
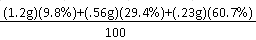

Comments
Post a Comment