Exploring Reactivity & Periodicity
Exploring Reactivity & Periodicity
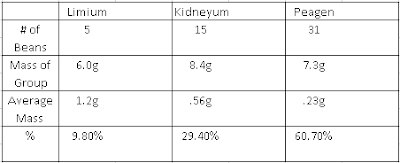
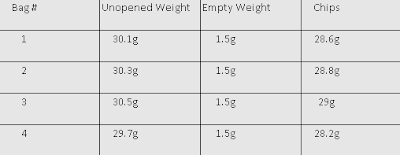
The question we were trying to answer was "Is reactivity a periodic property?" The reason we were trying to answer this question was to determine if reactivity is a periodic or quasi-periodic property. During our investigation we were given sodium(Na), magnesium(Mg), potassium(K), copper(Cu), and calcium(Ca). We put Na, Mg in two separate beakers and put K, Cu, and Ca in three separate test tubes. Each beaker/test tube was filled with distilled water.After we let each metal sit in the DI water for a few seconds we put a drop of phenolpthalein into each one and observed the reactions each one produced.
Claim: Reactivity is a periodic property
Evidence:
Sodium: Color change, sizzles across the water. (2)
Calcium: Fizzed, made bubbles in the test tube, and produced heat. (3)
Magnesium: Fizzed, changed color. (4)
Copper: Nothing. (5)
Reasoning: Our reasoning is that the elements that are located more to the left of the periodic table are more reactive than the ones located to right. Na, K were located on the first column Na being on the third row and K being on the fourth row. Mg, CA were located on the second column Mg being on the third row and Ca being on the fourth row. Cu was located to the far right on column 11, fourth row. K was seen as the most reactive of the bunch and Cu being the least reactive. The location of an element on the periodic table determines how reactive it will be, Down to the left being the most reactive and up to the right being the least reactive.

Comments
Post a Comment