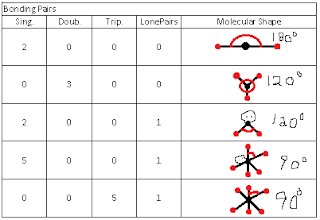
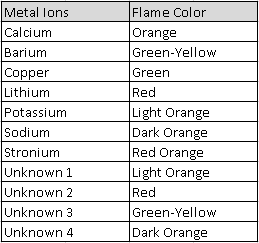
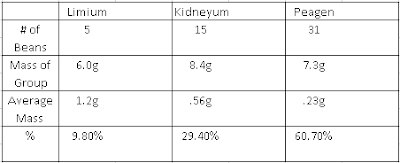
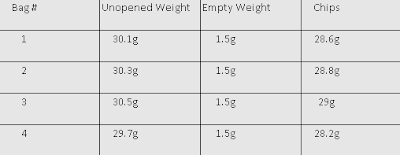
Exploring The Chemist's Dozen Lab
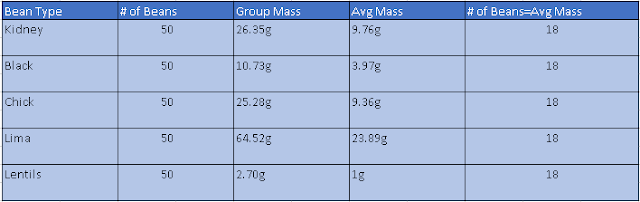
The Mole The question we were trying to answer was "Do average masses of different beans really equal a standard quantity." We were trying to answer this question to get a better understanding of Avogadro's Number and to see if the average of a different substance will equal a standard quantity. During our investigation we took 5 types of beans and massed each bean type as a group of 50. We measured the mass, relative mass, and the amount of beans equal to the relative mass and plotted them in a table. Our claim was yes, average masses of different beans do equal a standard quantity. The amount of beans are a standard quantity because they are all equal to 18 based on the average mass of the type of bean. Relative mass is the average mass, we divided the group mass of each bean type by the mass of the lentils. the mass of Lima beans are 23.09 times mire massive than the lentils mass. This models a mole because...