Exploring Atomic Structure & Electromagnetic Radiation
Exploring Atomic Structure & Electromagnetic Radiation
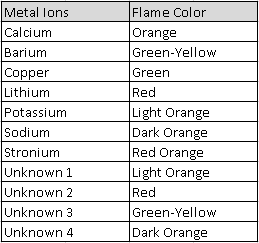
The question we were trying to answer was "What are the identities of the unknown solutions?" The reason we were trying to answer this question was to get a better understanding of the unique colors each one emits. During our investigation we had seven wood sticks soaked in the solutions CaCl, BaCl, CuCl, LiCl, KCl, NaCl, SrCl, and four soaked in a unknown solution. We took each solution soaked stick, tested them in a flame test and observed the colors each one produced.
Claim: Unknown 1: Potassium, Unknown 2: Lithium, Unknown 3: Barium, Unknown 4: Sodium
Evidence:
Reasoning: Our reasoning is that 1, 3, and 4 are different shades of orange and green. Ion 2 matched the color lithium produced when it was put under fire. A flame test is used to determine the metal ion based on the color it produces. It produces color because the electrons become excited by the energy of the flame, because of this the electrons move to a higher energy orbital then return to its ground state emitting the energy in the form of light.

Comments
Post a Comment